Teamwork in the inner ear – our hearing is based on the organized grouping of proteins
Researchers at Göttingen Campus have succeeded for the first time in examining the tiny synapses in the inner ear — the points of contact between the hair cells and the auditory nerve cells — at the molecular level. They were able to show that ion channels and other synaptic proteins essential for hearing are organized in specific patterns. This arrangement ensures optimized transmission of auditory information to the brain. These findings could contribute to the development of therapies for hearing disorders with synaptic causes. The results have been published in the journal Science Advances.
Published: 06.11.2025
Hearing is based on the conversion of sound into nerve signals. Sound-induced vibrations are converted into nerve impulses in the inner ear, which are transmitted to the brain via the auditory nerve and interpreted there as sounds, speech, or tones. The central hub of this process is the synapses — the points of contact between the hair cells and the auditory nerve cells. When the hair cells are stimulated by sound, calcium channels in the cell membrane open and calcium flows in. This leads to the release of a messenger substance that activates the receptors on the opposing auditory nerve cells, forming a nerve impulse.
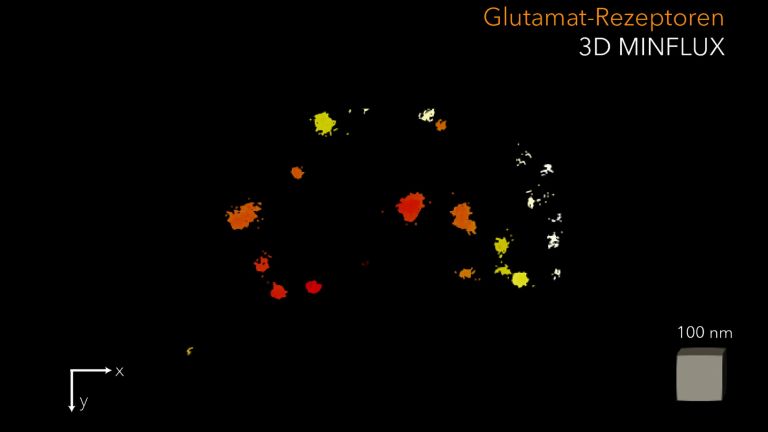
Scientists in Göttingen, led by the University Medical Center Göttingen (UMG), have been able to examine the tiny structure of synapses in inner hair cells for the first time. Using three-dimensional (3D) MINFLUX nanoscopy developed by Prof. Dr. Stefan Hell and colleagues at the Göttingen Campus, details in the range of a few nanometers, i.e., millionths of a millimeter, could be made visible. By optimizing sample preparation, the researchers were able to use this method to show that calcium channels and certain structural proteins in the hair cells organize themselves into small groups. These groups, also known as nanoclusters, are arranged in the form of stripes. The messenger substance is stored in tiny membrane vesicles, which the scientists were able to visualize for the first time at the synapse using MINFLUX. After being released into the gap between the hair cell and the auditory nerve cell, the messenger substance binds to the receptors of the opposing auditory nerve cell, which are arranged in the shape of a ring. This ring-shaped formation apparently allows for optimal detection of the released messenger substance. Using biophysical simulations, the researchers were able to demonstrate that this nanocluster arrangement enables highly efficient messenger substance release.
“The organized arrangement of calcium channels increases the likelihood of neurotransmitter release. These nanoclusters apparently enable us to perceive sounds precisely and quickly,” says Prof. Dr. Tobias Moser, director of the Institute for Auditory Neuroscience at UMG, spokesperson for the Cluster of Excellence “Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells” (MBExC) and spokesperson for the Collaborative Research Centre 1690 “Disease Mechanisms and Functional Restoration of Sensory and Motor Systems.” “This work provides the missing molecular map of the hair cell synapse and explains why it drives our fastest and most precise sensory system,” says Prof. Moser.
Original publication
Rohan Kapoor, Hyojin Kim, Evelyn Garlick, Maria Augusta do R. B. F. Lima, Klara Esch, Torben Ruhwedel, Wiebke Möbius, Fred Wolf, Tobias Moser. Charting the nanotopography of inner hair cell synapses using MINFLUX nanoscopy. Science Advances (2025). DOI: 10.1126/sciadv.ady4344